
Essential oils contain many different chemical constituents, all of which fall within the realm of Organic Chemistry.
As is commonly understood, all matter comprises atoms, which contain electrons that orbit around a nucleus. The nucleus, in turn, consists of protons with a positive electrical charge and electrically neutral neutrons. Each atom has an equal number of protons and electrons, resulting in a net electric charge of zero.
Electrons are arranged in layers surrounding the nucleus. Each of these layers has a maximum capacity for electrons. For instance, the first one can accommodate a maximum of two electrons, while the second layer can hold up to eight electrons, and so on. The electrons occupy the innermost layer initially, leaving gaps in the outermost layer of most atoms.
“Essential oils are extremely versatile materials; they are both medicine and fragrance. They can cure the most severe physical condition or reach to the depth of our souls.”
Lavabre, 1996
ESSENTIAL OIL CONSTITUENTS CAN BE CLASSIFIED ACCORDING TO THEIR FUNCTIONAL GROUP.
Such categories include monoterpenes, sesquiterpenes, alcohols, phenols, aldehydes, lactones, ketones, esters and ethers (phenolic and cyclic).
According to Joy Bowles and Robert Tisserand, studying the properties of constituents can be extremely useful, but these should be reviewed individually rather than in (functional) groups.
CHEMICAL COMPOUNDS: Monoterpenes
Among the functional groups, monoterpenes are the most volatile constituents. Indeed, they can evaporate before they have a chance to penetrate the skin. Therefore, they are classified as top notes in the perfume industry. Aromatically, they can smell like woody pine (from pinenes), citrus (from limonene), or medicinal resin (from terpinenes).
As with most essential oil constituents, monoterpenes are not soluble in water, as they are hydrocarbons with long non-polar carbon chains. However, they are soluble in vegetable oils and relatively soluble in ethanol.
MONOTERPENES HAVE ANTISEPTIC PROPERTIES YET ARE POSSIBLE SKIN IRRITANTS.

Examples:
- Limonene
- Pinene
- Myrcene
- Terpinene
- P-cymene
In Ayurveda, monoterpenes are classified as hot and dry. Therefore, although they do not correspond precisely to any energetic dosha, they could be a minor irritant to pitta dosha due to their heat and to vata dosha due to their dryness.
Miller, Light & Bryan, 1996
ESSENTIAL OILS CONTAINING HIGH PERCENTAGES OF MONOTERPENES:
CHEMICAL COMPOUNDS: Sesquiterpenes
Due to their molecular weight and higher boiling point, sesquiterpenes are less volatile than monoterpenes, making them a popular choice as a middle base note in perfumery. Furthermore, their significant molecular size results in lower ethanol solubility compared to monoterpenes, and they are also not soluble in water but quickly disperse in other oils and non-polar solvents.
SESQUITERPENES HAVE ANTI-INFLAMMATORY, ANTIVIRAL, ANTIALLERGENIC AND COOLING PROPERTIES.

Examples:
- Bisabolene
- b-Himachalane
- Caryophyllene
- Chamazulene
- Farnesene
In Ayurveda, sesquiterpenes correspond to the cold and dry categories. It means that, in theory, they produce Vata energy. However, sesquiterpenes can benefit all doshas due to their immunological properties.
Miller, Light & Bryan, 1996
ESSENTIAL OILS CONTAINING HIGH PERCENTAGES OF SESQUITERPENES:
- Black Pepper
- Myrrh
- Cedarwood (Atlas)
- German Chamomile
- Ginger

CHEMICAL COMPOUNDS: Alcohols
MONOTERPENOLS
Monoterpenols belong to the large family of monoterpenes and are composed of two isoprenyl units (which form 10-carbon skeletons). Consequently, this extended carbon chain skeleton renders them more soluble in ethanol, oils, and other non-polar solvents. However, due to their polar group, they exhibit a slight degree of solubility in water, typically ranging from 0.1-0.4 g/l.
In addition, monoterpenols are less volatile than monoterpenes and, thus, have higher boiling points. Indeed, this property makes them a preferred choice for middle notes in perfumery.
MONOTERPENOLS HAVE ANTISEPTIC, BACTERICIDAL, ANTIVIRAL, DIURETIC AND IMMUNE-STIMULATING PROPERTIES.

Examples:
- Geraniol
- Linalool
- Menthol
- Terpineol-4-ol
- Borneol
Monoterpenols do not exhibit any significant toxicity.
Tisserand & Balacs, 1995
Overall, monoterpene alcohols have a warm and uplifting effect. However, it is worth noting that these compounds tend to undergo slow oxidation when exposed to air. For this reason, it is advisable to store essential oils containing these molecules away from air, light, and heat to maintain freshness.
ESSENTIAL OILS CONTAINING HIGH PERCENTAGES OF MONOTERPENOLS:
SESQUITERPENOLS
A sesquiterpenol is a sesquiterpene with an attached alcohol group. Like monoterpenols, they also have a long carbon chain, which makes them evaporate slowly compared to other essential oil molecules. Indeed, they are considered base notes in perfumery.
The oils containing high percentages of sesquiterpenols are all dense and woody-earthy-smelling. Also, they are soluble in alcohol and vegetable oils but not in water, unlike monoterpenols.
SESQUITERPENOLS HAVE BACTERICIDAL, ANTI-INFLAMMATORY AND ANTIALLERGENIC PROPERTIES.
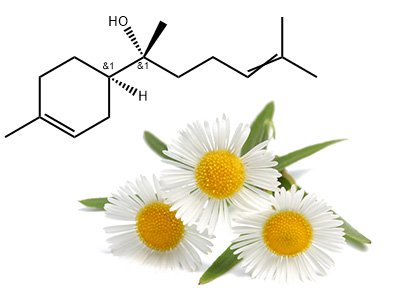
Examples:
- Bisabolol
- Farnesol
- Santalol
Based on Ayurvedic principles, sesquiterpene alcohols are highly moist but only slightly hot. Thereby, they may stimulate the Pitta dosha, albeit mildly.
Miller, Light & Bryan, 1996
ESSENTIAL OILS CONTAINING HIGH PERCENTAGES OF SESQUITERPENOLS:
CHEMICAL COMPOUNDS: Phenols
Phenols are told apart from the alcohols by the aromatic ring they have attached. Because of the electronegative effects of this benzene ring, their molecules become polar. Thus, they are slightly more soluble in water (1 g/l ) than hydrocarbons. Nevertheless, phenols do dissolve better in ethanol and other vegetable oils. Also, the boiling point of phenols is above 200 ° C. Therefore, they do not evaporate that fast and tend to have an acrid smell.
According to Bowles, phenols are more antibacterial and irritant to the skin and mucous membranes than other molecules with –OH groups.
Therefore, they could cause contact or sensitization dermatitis, and using them with appropriate dilution is recommended.
PHENOLS HAVE BACTERICIDAL, IMMUNE-STIMULATING, SKIN-IRRITATING, AND WARMING PROPERTIES.

Examples:
- Carvacrol
- Eugenol
- Thymol
- Chavicol
In Ayurveda, phenols stimulate Pitta dosha and are classified as warm and humid.
Miller, Light & Bryan, 1996
ESSENTIAL OILS CONTAINING HIGH PERCENTAGES OF PHENOLS:
- Bay Leaf
- Cinnamon Leaf
- Clove Bud
- Oregano
- Savoury
- Thyme

CHEMICAL COMPOUNDS: Aldehydes
Aldehydes derive from their parent’s primary alcohols. The carbonyl group is distinguished for having a double-bonded oxygen atom. Monoterpenoid aldehydes are as volatile as monoterpenols, and most smell more potent than their equivalent alcohol.
The carbonyl group is generally polar, making aldehydes slightly soluble in water. However, they are also soluble in ethanol and other oils, although they tend to be unstable and oxidize rapidly in the presence of oxygen. Therefore, unsurprisingly, they are considered irritating to the skin.
ALDEHYDES HAVE ANTI-INFLAMMATORY, SOOTHING AND ANTI-FUNGAL PROPERTIES.

Examples:
- Citronellal
- Geranial
- Neral
In Ayurveda, essential oils containing high aldehydes influence Kapha dosha (cold and moist).
Miller, Light & Bryan, 1996
ESSENTIAL OILS CONTAINING HIGH PERCENTAGES OF ALDEHYDES:
- Cinnamon
- Citronella
- Eucalyptus Lemon
- Lemongrass
- Melissa

CHEMICAL COMPOUNDS: Ketones
There are monoterpenoid and sesquiterpenoid ketones, although monoterpenoid ketones are the most common. They both share a peppermint-camphor scent, which feels quite “pungent” when you smell it. Ketones derive from secondary alcohols and are slightly soluble in water due to the presence of the polar carbonyl group. However, they are also soluble in ethanol and other oils.
Ketones are moderately stable but can cause problems in the body because they fight against being metabolized by the liver.
Tisserand & Balacs, 1995
KETONES CAN PROMOTE TISSUE FORMATION AND ARE MUCOLYTIC. HOWEVER, THEY ARE POTENTIALLY NEUROTOXIC.
Although single doses are unlikely to cause blood problems, their repeated use can lead to accumulation in the body due to the long half-life of terpenoids.

Examples:
- Camphor
- Pinocamphone
- Pulegone
- Thujone
- Verbenone
In Ayurveda classification, these compounds are also cold and moist, although they are slightly warmer than aldehydes. In theory, ketones produce kappa dosha energy. Therefore, they may aid in cell growth processes.
Miller, Light & Bryan, 1996
ESSENTIAL OILS CONTAINING HIGH PERCENTAGES OF KETONES:
- Camphor
- Caraway
- Hyssop
- Rosemary
- Sage
- Spearmint
- Yarrow

CHEMICAL COMPOUNDS: Esters
As esters are formed from alcohols and acids, they are named after both parent molecules, like ‘linalool + acetic acid’ that becomes ‘linalyl acetate.’
Although terpenoid acids are the most soluble in water among essential oil constituents (about 2g/l), esters are not. This probably happens because the two non-polar carbon chains neutralize the potentially polar part of their structure.
ESTERS HAVE SPASMOLYTIC, SOOTHING, AND ANTI-FUNGAL PROPERTIES.

Examples:
- Geranyl acetate
- Linalyl acetate
- Methyl salicylate
- a-Terpinyl acetate
Esters are relatively stable, particularly in essential oils with no water available for hydrolysis. Indeed, chemically, they are the most neutral components of essential oils. Therefore, Esters are highly appreciated for balancing doshas and are known as harmonizers.
According to Pénoël and Franchomme (1990), esters regulate and re-equilibrate the sympathetic nervous and neuroendocrine systems. In particular, he suggests these are the most significant properties of esters.
ESSENTIAL OILS CONTAINING HIGH PERCENTAGES OF ESTERS:
- Clary Sage
- Lavandin
- Lavender
- Myrtle
- Petitgrain
- Roman Chamomile
- Wintergreen
- Cardamom

CHEMICAL COMPOUNDS: Phenyl Methyl Ethers
Phenyl methyl ethers derive from phenols. They tend to have a strong, sweet smell and can be perceived as very low percentages in oils.
Most Ethers are soluble in ethanol but negligibly soluble in water. They tend to have boiling points below 200 ° C and are thus reasonably volatile compared to their cousins, the lactones and coumarins. In addition, ethers are not very reactive when exposed to heat and light, as both the methyl group and the benzene ring resist oxidation.
ETHERS ARE PREDOMINANTLY SPASMOLYTIC AND CARMINATIVE.
However, in large oral doses, they may lead to convulsions and even death, according to Tiserand & Balacs (1995).
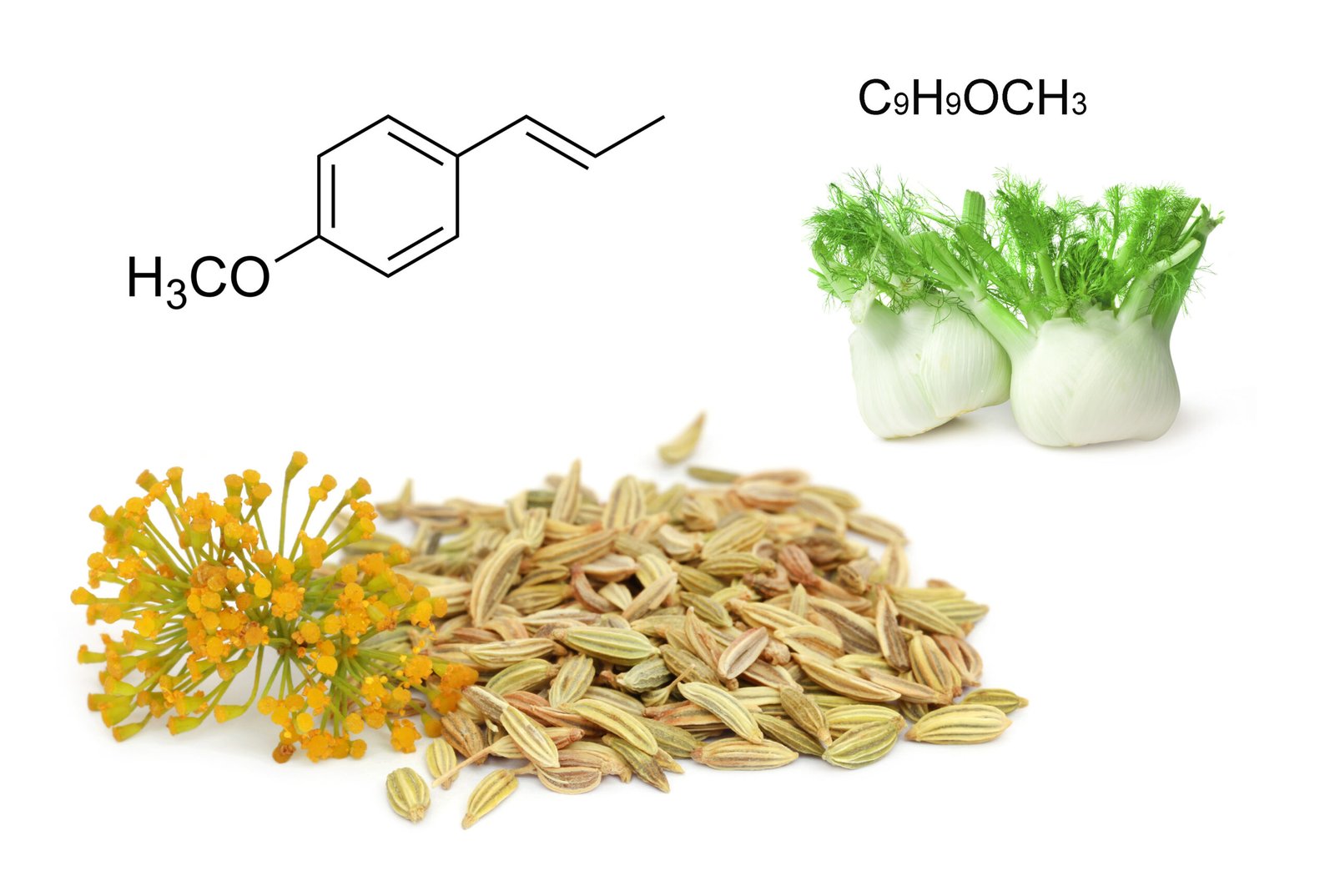
Examples:
- Trans-anethole
- Methyl chavicol
Ayurvedically, Ethers are somewhat more heating than Esters, and their harmonizing effects have applications to all doshas.
Miller, Light & Bryan, 1996
ESSENTIAL OILS CONTAINING HIGH PERCENTAGES OF ETHERS:
- Anise
- Basil
- Sweet Fennel
- Star Anise
- Tarragon

CHEMICAL COMPOUNDS: Cyclic Ethers (Oxides)
Monoterpenoid oxides are probably as volatile as monoterpenols. However, sesquiterpenoid oxides have similar volatility to sesquiterpenols.
Cyclic ethers are comparable to phenyl methyl ethers, but the oxygen atom is incorporated in a ring form rather than being sandwiched by a short carbon chain. In addition, oxides are barely soluble in water but dissolve well in ethanol and other oils.
Among the functional groups, the oxides are possibly the strongest odorants.
However, they break down into alcohol under heat and light conditions. Regarding volatility, monoterpenoid oxides are probably as volatile as monoterpenols, while sesquiterpenoid oxides have similar volatility to sesquiterpenols.
OXIDES ARE EXPECTORANTS AND HAVE IMMUNE-STIMULATING PROPERTIES.
In particular, not much research has been done on oxides, except for 1,8-cineole (Eucalyptol). However, Bowless mentions linalool oxide and 1,8-cineole could stimulate the respiratory tract.
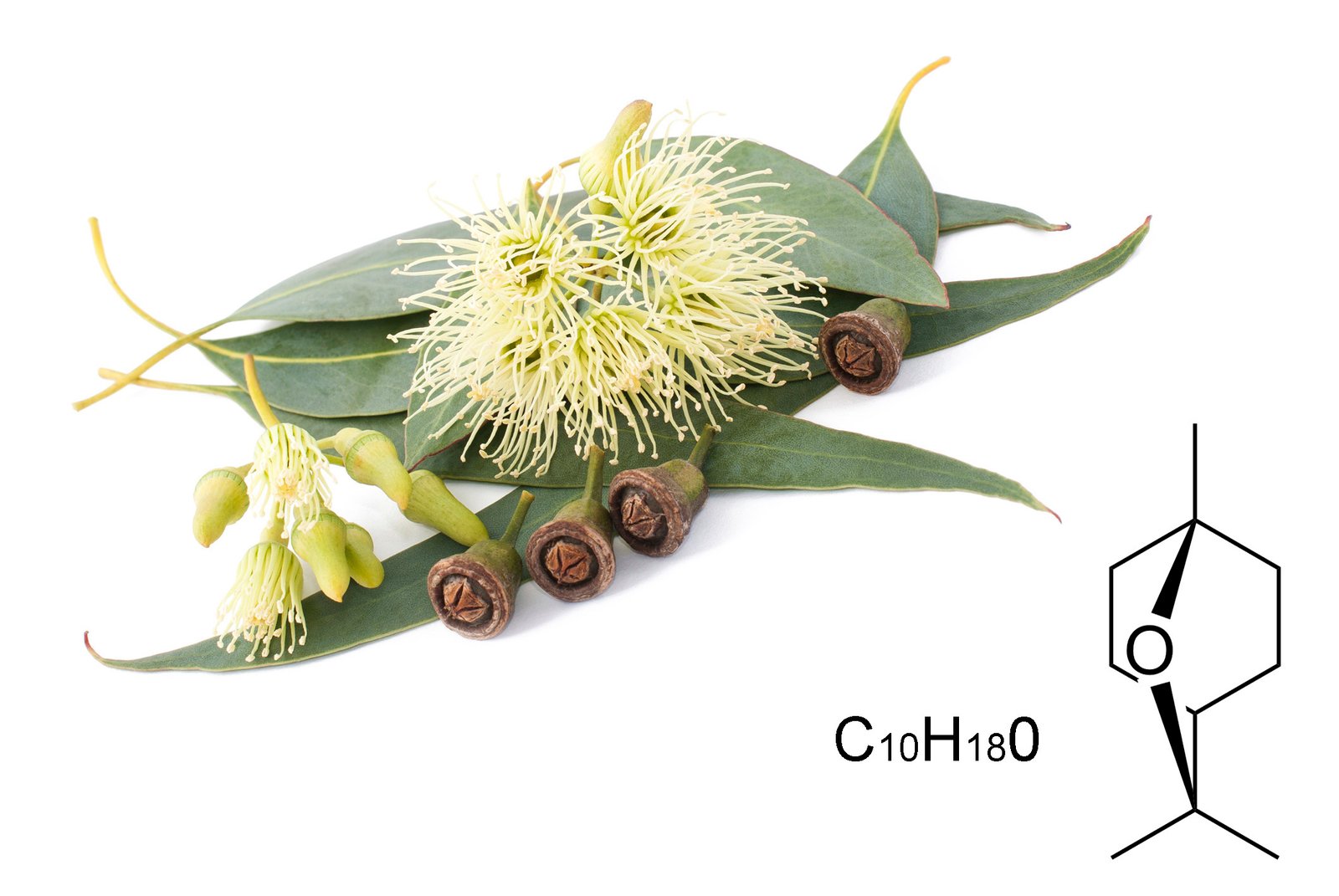
Examples:
- 1,8-cineole
- 1,4-cineole
Essential oils with camphor (ketones), menthol (phenols), and 1,8 cineole (oxides) are traditionally the ones most often utilized for decongesting the lungs and sinuses throughout colds, flu, bronchitis, pneumonia, asthma and other congestive conditions.
David Stewart, 2010
ESSENTIAL OILS CONTAINING HIGH PERCENTAGES OF 1,8-CINEOLE:
- Cajeput
- Cardamom
- Eucalyptus
- Myrtle
- Niaouli
- Rosemary
- Spike Lavender

CHEMICAL COMPOUNDS: Lactones
Lactones do not appear as the main components in any oil used in aromatherapy. However, like most other essential oil constituents, they are also negligibly soluble in water.
Sesquiterpenoid lactones are less volatile than monoterpenoid ones. Besides, they often have earthy and acrid odours that stay long. Notably, they last longer in the body because they are probably not metabolized that quickly.
LACTONES HAVE MUCOLYTIC AND PHOTOTOXIC PROPERTIES.

Examples:
- Nepetalactone
“Despite some lactones being allergens for some people, other research shows that sesquiterpenoid lactones have mighty anti-inflammatory properties.”
Mazor et al. (2000)
ESSENTIAL OILS WITH HIGH PERCENTAGES OF LACTONES:
- Catnip
According to Bowles, E Joy, skin allergies, sensitization, and blistering are the main caution of oils with high percentages of lactones. Fortunately, they only exist in small amounts in essential oils.

References
Beverley Hawkins, West Coast Institute of Aromatherapy Aromatherapy 201, 2009
Dr. Miller L. & Dr. Miller B., Ayurveda & Aromatherapy: The Earth Essential Guide to Ancient Wisdom and Modern Healing, Lotus Press, 1996.
E. Joy Bowles, Chemistry of Aromatherapeutic Oils, Allen & Unwin Academic, 3rd Edition 2003.
Kurt Schnaubelt, Ph.D., Advanced Aromatherapy: The Science of Essential Oil Therapy, Healing Arts Press, 1998.
Marcel Lavabre, Aromatherapy Workbook, Healing Arts Press, 1996.
Tisserand R, Balacs T. Essential oil safety: A guide for health care professionals. Edinburgh (GB): Churchill Livingstone, 1995.




