
Have you ever wondered about the extraction methods of essential oils?
Let’s start by saying that aromatic compounds are extracted from plant materials, so we can not just “make” them. A standard essential oil consists of hundreds of chemical constituents, and the plant has created each one.
They are obtained from raw materials such as flowers (rose, jasmine, ylang-ylang), leaves (mint, rosemary, eucalyptus), fruits (lemon, bergamot, grapefruit), seeds (fennel, cumin, coriander), grass (lemongrass, palmarosa), roots (ginger, vetiver), rhizomes (radish, calamus), woods (sandalwood, rosewood, cedarwood), bark (cinnamon), gums (incense), bulbs (garlic), and dried flower buds (Clove).
We need considerable plant material to produce a single essential oil bottle, underscoring its high concentration.
Numerous methodologies exist for extracting essential oils from plants, each with its own unique set of benefits. These methods are commonly used by professionals in aromatherapy and essential oil production to safely and effectively extract the desired compounds from plants.
- Steam and Water Distillation
- Enfleurage
- Cold Press (Expression)
- CO2 extraction
- Solvent Extraction
All the above methods aim to obtain a plant’s active botanical components, its “life force.” However, each of them will be useful depending on the part of the plant involved in the extraction process.
EXTRACTION METHODS: Steam Distillation
The steam-distilled process is the most common and effective method for extracting essential oils from plants. This process involves subjecting the plant’s raw material to high temperatures, which prompts the release of the plant’s aromatic molecules via the steam.
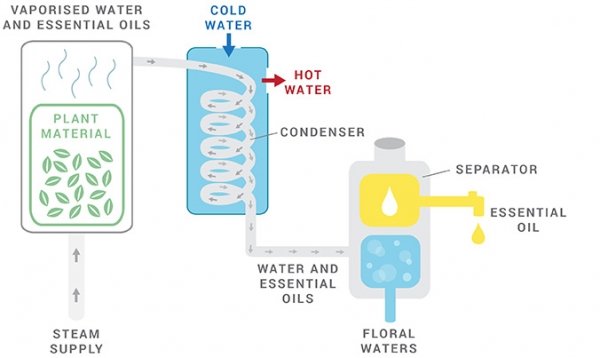
This distillation technique is particularly efficient and reliable on temperature-sensitive botanical matter.
- First, it is necessary to spread highly purified and odourless vegetable or animal fat, generally lard or tallow, on glass plates (Chassis) and let it set
- By injecting vapour, the botanical matter releases the plant’s aromatic molecules. Then, these vaporized compounds travel to the condensing flask. A condenser commonly has a separate tube or outer chamber through which cold water circulates to provide more effective cooling. As a result, the vapour cools and turns into a liquid.
- The aromatic liquid by-product falls from the condenser and collects inside a vessel below it, called a separator. Since water and oil don’t mix, the essential oil floats on top of the water. From here, it deviates.
EXTRACTION METHODS: Water Distillation
Delicate botanicals like orange blossoms or roses are materials that tend to clump during steam distillation.

In such cases, it is best to immerse the plant matter in boiling water to optimize extraction. By doing so, the water serves as a protective barrier and prevents the extracted oil from overheating. Ultimately, the liquids condense, cool off, and then separate from each other.
Interestingly, an often-overlooked by-product of this process is floral water. This hydrosol, hydrolate water, floral water, or herbal distillate contains trace amounts of the plant’s molecules, providing subtle yet powerful therapeutic properties. One example is rose water, widely used for its skin-hydrating and calming effects.
EXTRACTION METHODS: Enfleurage
Enfleurage has a rich history in the perfume industry because it helps capture the fragrances of delicate flowers. (Such as jasmine, orange blossom, and tuberose). The process can be either “cold” or “hot.” In both circumstances, the fat saturated with fragrance results in “enfleurage pomade.”
Cold Enfleurage originated in the 18th century in southern France.
COLD ENFLEURAGE
- First, it is necessary to spread highly purified and odourless vegetable or animal fat, generally lard or tallow, on glass plates (Chassis) and let it set.
- Then, we placed fresh petals or whole flowers on top of the layer of fat and pressed in. Afterward, depending on the flowers we used, we allowed it to set for 1-3 days or a couple of weeks. During this time, their scent seeped into the fat.
- We can repeat the process until the fat reaches the desired saturation, replacing the withered petals with fresh ones.
- The final product is the enfleurage pomade: the fat and the fragrant oil. Then, it is washed with alcohol to separate the botanical extract from the remaining fat, which is used to make soap. When the alcohol evaporates from this mixture, the “absolute” is what is left over.
HOT ENFLEURAGE
- The only difference in this process is that the fats are heated.
Unlike the cold process, in hot Enfleurage, the solid fats must be heated first before adding the botanical matter. Then, the spent flowers are repeatedly strained from the fat and replaced with fresh material until the grease is saturated with the scent.
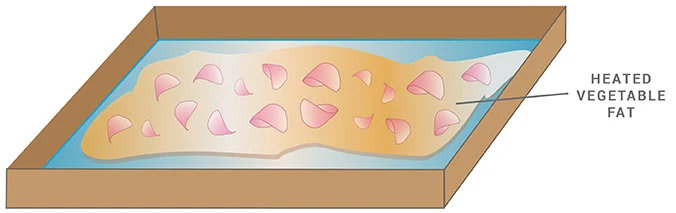
Hot Enfleurage is the oldest procedure for preserving plant fragrance substances.
Although this method is no longer commercially viable, some Grasse, France distilleries still use this technique to produce high-quality concentrates.
EXTRACTION METHODS: COLD-PRESSED
Cold-pressed extraction, or Expression, is mainly used for citrus peels. Since no heat is used in this procedure, the aroma and chemical structure of expressed oils are almost identical to those within the fruit’s skin.
Unlike distilled oils, expressed essences contain non-volatile substances such as waxes.
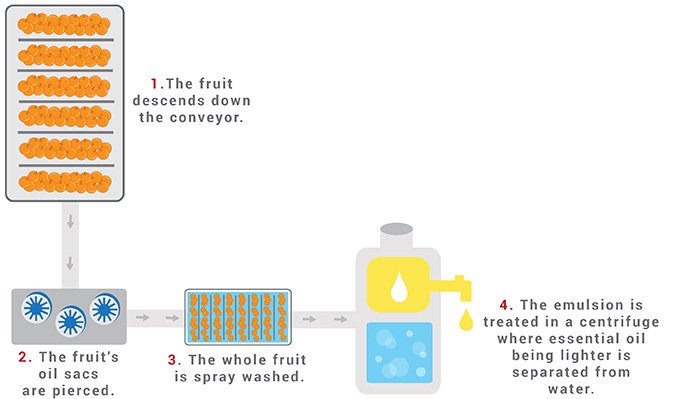
As a result, they have a relatively short shelf life. The oils will deteriorate within six to nine months, whereas most distilled essences last up to two years.
- First, the whole fruit is placed in a rotating drum that mechanically pierces it to break the essential oil sacs inside the peel.
- Once the botanical material is in the collection area, it is pressed to squeeze out the juice and oil.
- The oil and juice produced still contain solids from the fruits, such as the peel. Thus, it must be centrifuged to filter the solids from the liquids.
- The oil is separated from the juice layer and diverted to another container.
EXTRACTION METHODS: Carbon Dioxide (CO2)
CO2 extraction is a promising new technique for extracting aromatic compounds from raw materials. It utilizes supercritical CO2 as the extraction solvent, which offers several advantages over traditional methods. Indeed, this technique allows for more precise and efficient extraction, reducing waste and improving product quality.
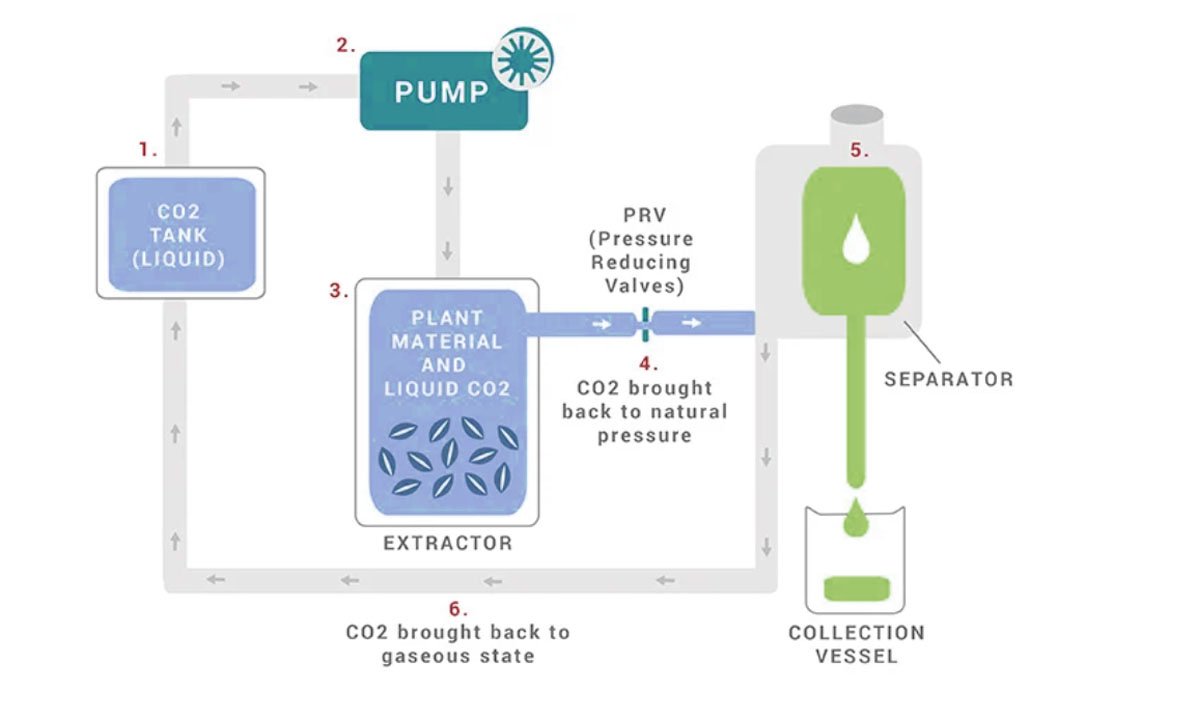
CO2 extracts are typically thicker than steam distiller essential oils.
The extraction takes minutes, and there are no chemical reactions between the solvent and aromatic substances. Also, since CO2 is gas at normal atmospheric pressure, it leaves no trace of itself in the final product. The Carbon Dioxide extraction method is safer and environmentally friendly, making it a popular choice for many industries.
CO2 extracts tend to have a stronger aroma of the natural plant, but they can be quite expensive and not always easy to find.
- Under normal pressure, CO2 changes directly from a solid to a gas in a process known as sublimation. However, when carbon dioxide is subjected to high pressure slightly above room temperature, a “supercritical” fluid is formed.
- Due to the liquid properties of the gas, CO2 works as a natural solvent, extracting pigments and resins from plant matter.
- When the CO2 returns to natural pressure, it evaporates into its gaseous state, remaining behind the essential oil.
EXTRACTION METHODS: Solvent Extraction
This method employs food-grade solvents like hexane and ethanol to treat the botanical material. Indeed, this technique is especially advantageous for plants that exhibit low oil production or contain significant resin. Additionally, it is handy for extracting delicate aromatics that cannot withstand the pressure and distress of steam distillation.
Solvent extraction produces a finer fragrance than any other type of distillation method.

Although this method produces a more delicate scent, we should remember that the chemicals mentioned above used in the process remain in the oil. Thus, anyone allergic to the solvents could have a reaction if rubbed on the skin.
- Adding the solvent to plant material produces a waxy aromatic compound called “concrete.”
- Then, this wax and oil solution goes through a low-pressure distillation process to remove the non-volatile plant material, such as waxes and pigments.
- When this concrete substance is mixed with alcohol, the oil particles are released.
- Once carbon dioxide reverts to its inherent pressure, it becomes gaseous, with the aromatic oil remaining as a residual substance.
References
Beverley Hawkins, West Coast Institute of Aromatherapy Aromatherapy 201, 2009
Bettina Malle & Helge Schmickl, The Essential Oil Maker’s Handbook: Extracting, Distilling & Enjoying Plant Essences, Spikehorn Press, 2015
Madeleine Kerkhof, CO2 Extracts in Aromatherapy: 50+ Extracts for Clinical Applications, De Levensboom Complementaire Opleidingen, 2018
Salvatore Battaglia, The Complete Guide to Aromatherapy, The Perfect Potion (Aust) Pty Ltd, Virginia, Q, Australia, 1997

